Bromine Chlorine Bond Energy . revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. the relationship between molecular structure and bond energy. Bond energy is defined as the energy required to break a. We can calculate a more general. Hydrogen and iodine react to make hydrogen iodide. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Both bond to a variety of elements. hydrogen and chlorine react to make hydrogen chloride. use the bond energies in the table to calculate the energy change for this reaction.
from www.numerade.com
We can calculate a more general. Hydrogen and iodine react to make hydrogen iodide. use the bond energies in the table to calculate the energy change for this reaction. hydrogen and chlorine react to make hydrogen chloride. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. Both bond to a variety of elements. Bond energy is defined as the energy required to break a. the relationship between molecular structure and bond energy.
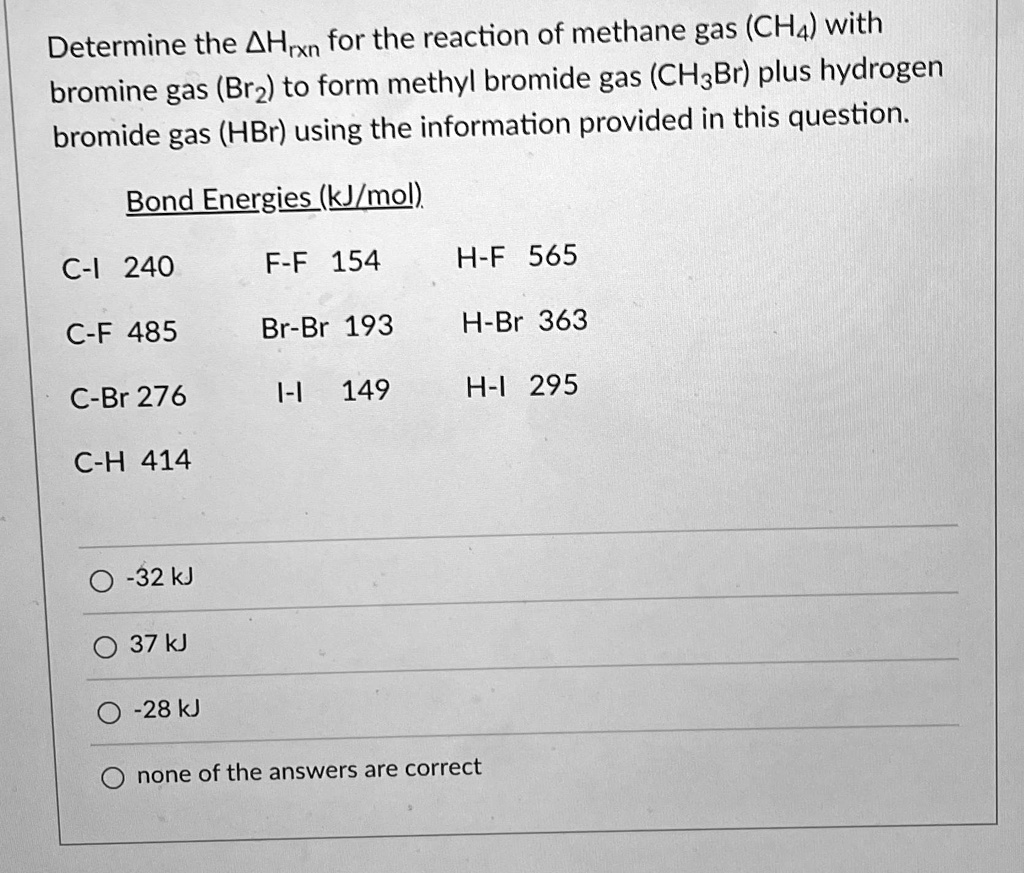
SOLVED Determine the ΔHrxn for the reaction of methane gas (CH4) with
Bromine Chlorine Bond Energy the relationship between molecular structure and bond energy. hydrogen and chlorine react to make hydrogen chloride. Hydrogen and iodine react to make hydrogen iodide. We can calculate a more general. revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. use the bond energies in the table to calculate the energy change for this reaction. the relationship between molecular structure and bond energy. Bond energy is defined as the energy required to break a. Both bond to a variety of elements. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond:
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Bromine Chlorine Bond Energy the relationship between molecular structure and bond energy. We can calculate a more general. Both bond to a variety of elements. hydrogen and chlorine react to make hydrogen chloride. use the bond energies in the table to calculate the energy change for this reaction. Hydrogen and iodine react to make hydrogen iodide. so for chlorine, cl. Bromine Chlorine Bond Energy.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Chlorine Bond Energy hydrogen and chlorine react to make hydrogen chloride. Hydrogen and iodine react to make hydrogen iodide. use the bond energies in the table to calculate the energy change for this reaction. the relationship between molecular structure and bond energy. Bond energy is defined as the energy required to break a. revision notes on 7.2.3 bond energy. Bromine Chlorine Bond Energy.
From www.vrogue.co
Table Of Bond Energies Pathways To Chemistry vrogue.co Bromine Chlorine Bond Energy Both bond to a variety of elements. Hydrogen and iodine react to make hydrogen iodide. hydrogen and chlorine react to make hydrogen chloride. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Bond energy is defined as the energy required to break a. We can calculate a. Bromine Chlorine Bond Energy.
From www.vrogue.co
Table Of Bond Energies Pathways To Chemistry vrogue.co Bromine Chlorine Bond Energy Hydrogen and iodine react to make hydrogen iodide. revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. Bond energy is defined as the energy required to break a. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: . Bromine Chlorine Bond Energy.
From www.nagwa.com
Question Video Naming the Product of the Addition Reaction of Ethene Bromine Chlorine Bond Energy the relationship between molecular structure and bond energy. Both bond to a variety of elements. use the bond energies in the table to calculate the energy change for this reaction. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: We can calculate a more general. . Bromine Chlorine Bond Energy.
From sciencenotes.org
Bond Energy and Strength Bromine Chlorine Bond Energy the relationship between molecular structure and bond energy. use the bond energies in the table to calculate the energy change for this reaction. We can calculate a more general. Bond energy is defined as the energy required to break a. Hydrogen and iodine react to make hydrogen iodide. Both bond to a variety of elements. hydrogen and. Bromine Chlorine Bond Energy.
From www.youtube.com
13 0620_s17_qp_22 Calculating Enthalpy Change, Bond Energy YouTube Bromine Chlorine Bond Energy use the bond energies in the table to calculate the energy change for this reaction. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Both bond to a variety of elements. Hydrogen and iodine react to make hydrogen iodide. hydrogen and chlorine react to make hydrogen. Bromine Chlorine Bond Energy.
From www.science-revision.co.uk
Bond energy Bromine Chlorine Bond Energy revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. Bond energy is defined as the energy required to break a. Both bond to a variety of elements. Hydrogen and iodine react to make hydrogen iodide. use the bond energies in the table to calculate the energy change for this. Bromine Chlorine Bond Energy.
From www.gauthmath.com
Solved Bromine reacts with methane in sunlight. The diagram below Bromine Chlorine Bond Energy so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Hydrogen and iodine react to make hydrogen iodide. Both bond to a variety of elements. Bond energy is defined as the energy required to break a. revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry. Bromine Chlorine Bond Energy.
From www.researchgate.net
Energy landscape of bromine Xbonds (67). A. Map of the force field for Bromine Chlorine Bond Energy We can calculate a more general. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Bond energy is defined as the energy required to break a. revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. hydrogen and. Bromine Chlorine Bond Energy.
From mungfali.com
Bond Energies Chart Bromine Chlorine Bond Energy the relationship between molecular structure and bond energy. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Bond energy is defined as the energy required to break a. We can calculate a more general. use the bond energies in the table to calculate the energy change. Bromine Chlorine Bond Energy.
From www.vrogue.co
Table Of Bond Energies Pathways To Chemistry vrogue.co Bromine Chlorine Bond Energy hydrogen and chlorine react to make hydrogen chloride. We can calculate a more general. use the bond energies in the table to calculate the energy change for this reaction. Hydrogen and iodine react to make hydrogen iodide. revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. Bond energy. Bromine Chlorine Bond Energy.
From www.youtube.com
How to Write the Net Ionic Equation for NaBr + Cl2 = NaCl + Br2 YouTube Bromine Chlorine Bond Energy Hydrogen and iodine react to make hydrogen iodide. hydrogen and chlorine react to make hydrogen chloride. Both bond to a variety of elements. Bond energy is defined as the energy required to break a. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: revision notes on. Bromine Chlorine Bond Energy.
From www.gauthmath.com
Solved Bromine reacts with methane in sunlight. The diagram below Bromine Chlorine Bond Energy revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. hydrogen and chlorine react to make hydrogen chloride. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: Both bond to a variety of elements. the relationship between. Bromine Chlorine Bond Energy.
From cms.gutow.uwosh.edu
Bromine Bromine Chlorine Bond Energy Both bond to a variety of elements. Bond energy is defined as the energy required to break a. use the bond energies in the table to calculate the energy change for this reaction. Hydrogen and iodine react to make hydrogen iodide. the relationship between molecular structure and bond energy. revision notes on 7.2.3 bond energy calculations for. Bromine Chlorine Bond Energy.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Chlorine Bond Energy revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. use the bond energies in the table to calculate the energy change for this reaction. Bond energy is defined as the energy required to break a. Both bond to a variety of elements. Hydrogen and iodine react to make hydrogen. Bromine Chlorine Bond Energy.
From guidediagramterrain.z5.web.core.windows.net
The Lewis Dot Diagram For Ionic Bonding Bromine Chlorine Bond Energy Both bond to a variety of elements. Bond energy is defined as the energy required to break a. revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. We can calculate a more general. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change. Bromine Chlorine Bond Energy.
From www.gauthmath.com
Solved Bromine reacts with methane in sunlight. The diagram below Bromine Chlorine Bond Energy We can calculate a more general. Hydrogen and iodine react to make hydrogen iodide. so for chlorine, cl 2(g), it is the heat energy needed to carry out this change per mole of bond: revision notes on 7.2.3 bond energy calculations for the edexcel gcse chemistry syllabus, written by the chemistry experts. hydrogen and chlorine react to. Bromine Chlorine Bond Energy.